South Alabama Ionics Liquid Laboratory
Posted on December 16, 2020 by USA College of Engineering

Fall 2020 marks the second year of work for the Alabama Advanced Solvents Cluster (AASC) which is led by the South Alabama Ionic Liquids Laboratory (SAILL). SAILL is made up of faculty from the Chemical Engineering and Chemistry departments at the University of South Alabama and AASC includes faculty researchers from the University of Alabama, Tuskegee University, and the University of North Alabama. Kevin West, USA Professor of Chemical Engineering is scientific lead on the project on which Associate Professor Christy Wheeler West and Assistant Professor Brooks Rabideau are senior personnel. The cluster was formed in 2019 to carry out a two-year, $2.7 million EPSCoR award from the Department of Energy. A major focus of the project is to identify the fundamental, molecular-level interactions between thermally robust ionic liquids (non-volatile salts which are liquids at temperatures where they are used) and the molecular compounds dissolved in them, specifically focusing on how these interactions can be optimized for environmentally benign and energy efficient chemical processes.
The cluster is using a combination of chemical synthesis, experimental characterization, and molecular simulation to understand how these unique solvents can be used to improve chemical separations, reactions, and polymer synthesis and processing. One target application is the efficient separation of aromatic compounds from petroleum streams, an industrially important process that is currently very energy intensive. Understanding how and why the ionic liquids interact more strongly with the aromatic species will enable the design of effective and industrially practical separations. Current work in this area is focused on the measurement and simulation of phase behavior related to these processes. Another aspect of the work is investigating the ability of modified versions of the ionic liquids to act as self-neutralizing acids, becoming strongly acidic only at high temperature. Industrially, acids such as sulfuric acid and other hazardous and volatile acids are used as catalysts in large volumes. Though effective, they are often not recyclable as they must be neutralized after use, resulting in the generation of large amounts of waste. The self-neutralizing ionic liquids studied in this work have the potential to provide the needed acidity for catalytic activity while remaining non-volatile and reusable. Additional potential uses of these ionic liquids include their use as high-temperature heat transfer fluids for renewable energy applications such as solar thermal energy generation and storage.
-
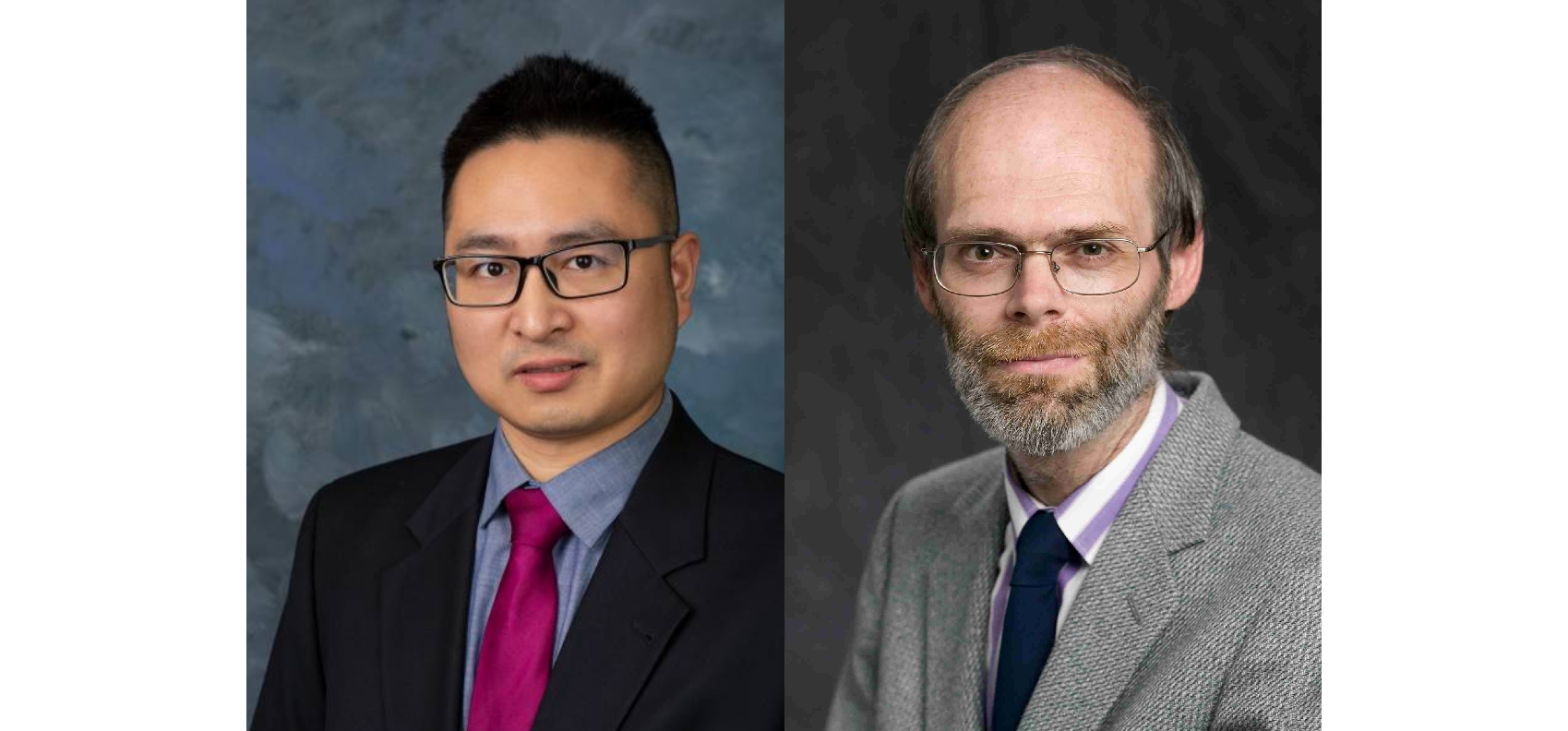
Dr. Woods and Dr. Wu honored by MACE awards
This year, two prominent faculty from the College of Engineering are r...
March 3, 2025 -

Launching a Career
Launching a Career...
August 8, 2024 -

USA Engineering Professor Receives New NSF Grant for Novel AI Computing Systems
A new $275,000 project entitled "Carbohydrate Memristor Empowered...
August 13, 2024 -

South Launches New Faculty Ambassador Program
South Launches New Faculty Ambassador Program...
July 30, 2024